Tulane testing new ‘drop-of-blood’ diagnostic device for trauma patients
Tulane University biomedical engineers are developing a new device to rapidly detect life-threatening blood-clotting problems in trauma patients using just a single drop of blood.
It’s a potentially life-saving advancement for cases of severe injury, where every minute counts as emergency teams work to stop bleeding and assess a patient’s coagulopathy, a condition that can cause excessive bleeding or blood clots due to defects in the body’s clotting process.
There is currently no reliable or fast way to perform such tests in emergency settings, said Damir Khismatullin, associate professor of biomedical engineering in Tulane’s School of Science and Engineering.
“In emergencies, patients are losing a lot of blood, and right now blood transfusion is done — truly speaking, blindly — to save their lives,” Khismatullin said. “However, improper blood transfusion is exactly what’s causing most of the coagulation problems." Last year, Levisonics Inc., a medical device startup Khismatullin co-founded in 2016 to commercialize the technology, received a $4.3 million grant from the U.S. Department of Defense (DoD) to test the device to make sure it works in real-world settings.
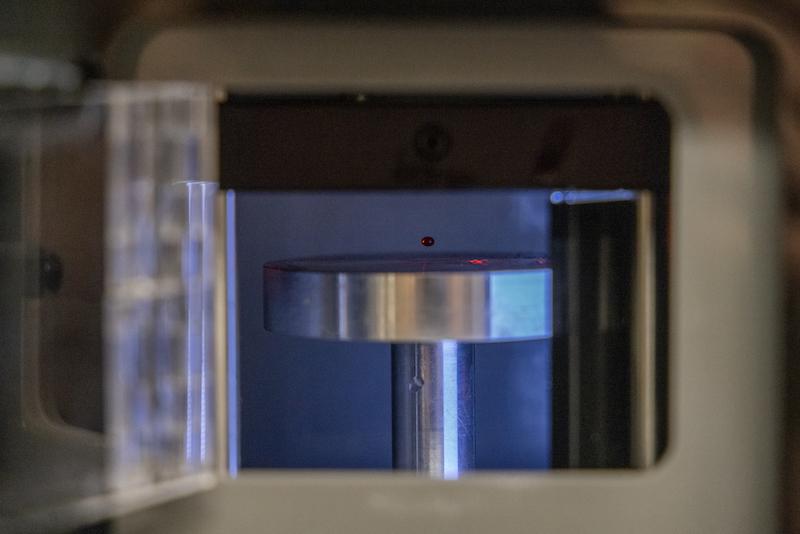
The testing phase began in July, shortly after the device was delivered to Tulane’s uptown New Orleans campus. Testing will take about a year and a half and includes helping to develop clinical protocols for managing trauma-induced coagulopathy using the new instrument — a battery-powered “acoustic tweezing device” designed for emergency use in combat zones, field hospitals and emergency rooms.
Roughly the size of a small microwave oven, the device is portable and will be able to operate without access to the electrical grid. Researchers anticipate it could be carried in a backpack and used on a stable surface, such as a field hospital table.
At Tulane, researchers are using finger-prick and venipuncture blood samples from healthy volunteers and modifying the samples to mimic the coagulopathy that occurs immediately after trauma and during transfusion therapy.
Currently, standard diagnostics for coagulation in trauma patients can take hours in major hospitals equipped with the necessary tools. In smaller or rural hospitals, blood coagulation analysis can take days — or even more than a week — when samples are sent to offsite labs. The new device aims to reduce that wait time to less than 20 minutes using a drop of blood from a finger prick or venipuncture.
In lay terms, the device levitates a drop of blood in the air using sound waves. The drop is gently squeezed by modulating those waves, allowing researchers to measure how the blood's physical properties change over time. This method is known as acoustic tweezing spectroscopy.
“We measure viscoelastic properties of blood from how the drop deforms in response to acoustic wave modulation,” Khismatullin said. “We repeat these measurements every 30 seconds to one minute, which allows us to assess the blood coagulation status.”
The technology builds on earlier versions developed in Khismatullin’s lab. The transparent prototype researchers demonstrated during a recent lab visit was an early, non-commercial version of the device, enclosed in a clear case for visibility. One of the goals of the current project is to compare performance between that prototype and the commercial-ready version from Levisonics.
The team at Tulane is working in close collaboration with researchers at Indiana University School of Medicine, where the device is being tested on blood samples from actual trauma patients. That data will help validate the device’s use for real-world trauma care.
“I am immensely grateful for their expertise and support, which has been instrumental in securing this grant,” said Nithya Kasireddy, president and CEO of Levisonics and the project’s principal investigator. “Together, we are poised to make meaningful advancements in point-of-care diagnosis and hemostasis management for trauma patients, and I am excited for the journey ahead.”
Although clinical trials have not yet begun, real-world applications of the acoustic tweezing technology are already underway. The team has used a related method, quasi-static acoustic thromboelastometry, to monitor blood coagulation in liver transplant patients and children on Extracorporeal Membrane Oxygenation, a life support system used in intensive care units for patients with life-threatening injuries and illnesses. The results of those studies have been submitted for publication.
Another current project, funded by a separate National Institutes of Health-supported subaward from Levisonics, is testing the technology for use in pediatric patients with hemophilia, an inherited genetic disorder that impairs the body’s ability to make blood clots. That work is being performed in partnership with the Louisiana Center for Bleeding & Clotting Disorders at Tulane University School of Medicine.
Tulane’s success metrics for the DoD-funded project will include demonstrating the Levisonics device’s performance in analyzing blood coagulation and its ability to monitor simulated trauma-related changes in the lab. The ultimate goal is to guide timely, targeted treatment for trauma patients in the field.
While military medics are not yet directly involved, Levisonics reports growing interest from the DoD for potential future deployment. Additional testing in extreme weather conditions, such as cold or high heat, will be conducted later with further Army support.
Before the device can be used widely in hospitals, it must receive clearance from the U.S. Food and Drug Administration. In the meantime, the technology is already available for research use by universities, labs and pharmaceutical companies.
“The development of this device has the potential to save lives and improve health outcomes for trauma patients in various emergency settings,” Khismatullin said. “It fills a critical gap where seconds matter, giving medics and clinicians the information they need to act quickly and precisely, and we’re hopeful this technology will become a standard tool in the future of trauma care.”