Tulane University, Children’s Hospital launch Moderna Covid-19 vaccine trial for children from 6 months to 11 years old
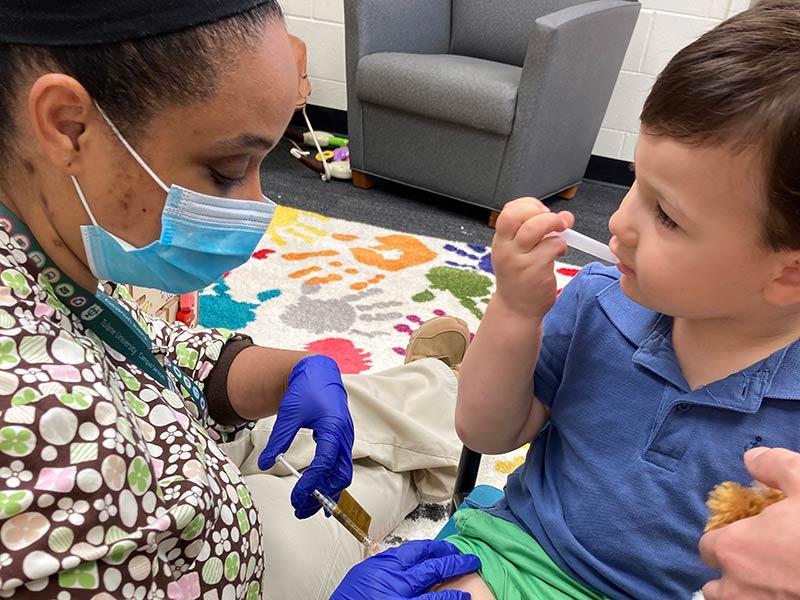
Tulane University School of Medicine and Children’s Hospital New Orleans are participating in a national clinical trial to test the effectiveness of Moderna’s COVID-19 vaccine in children ages six months to 11 years old.
The KidCOVE Study plans to enroll children across the country to test how the vaccine may protect young children from getting sick if they come into contact with SARS-CoV-2, the virus that causes COVID-19. Portions of the trial underway are also studying vaccine dosing levels to find out which is the safest and most effective for children.
“Having a safe and effective vaccine that works for everyone, including kids, is an important step towards protecting the whole family, and getting back to resuming the activities that we all miss,” said pediatrician Dr. Monika Dietrich, who is leading the trial at Tulane and Children’s Hospital. “Vaccination is the most important public health strategy right now to help fight this pandemic, especially as we are seeing surges in cases due to new variants of concern. Families who take part in the study will play an important role in helping us get closer to making sure everyone can get vaccinated.”
Moderna’s mRNA-1273 vaccine is being tested at three dosing levels to find out which works best for children. Many vaccines for viruses are made from a weakened or inactive virus, but the mRNA-1273 study vaccine is made from messenger ribonucleic acid (mRNA), which is an instructional molecule that naturally occurs in the body and tells cells how to make protein. The protein is a small part of the virus that helps the body’s immune system protect itself if exposed to SARS-CoV-2.
“To date, more than 30,000 adults and children over the age of 12 have taken part in the research for mRNA-1273.” Dietrich said.
Participants will have two injection visits, which will be about 28 days apart, and researchers will compare results to those who receive a placebo. There is a 75% chance participants will receive the study vaccine and a 25% chance they will receive the placebo.
This study lasts about 12 months after the last injection, and includes phone calls, e-diary entries, telemedicine visits, blood draws and nasal swabs.hildren and their parents will return for study visits three or four times in the year after the injection.
To participate, children must be:
- Between six months old and less than 12 years old and in good health.
- Not have a positive COVID-19 test within two weeks prior to receiving the first vaccination.
- Be free from exposure to someone with SARS-CoV-2 infection or COVID-19 within two weeks prior to receiving the first vaccination.
- Not have received an investigational vaccine or treatment for COVID-19.
The study is registering participants now with plans to begin enrollment in August. To register, visit www.KidCOVEstudy.com, check your child’s eligibility and select the New Orleans site. For questions about the study, parents may also email tumodernakidsvaccine@gmail.com or call 504-517-2215.
Enrollment will be done by age group. Registrants will be notified by email of updates on enrollment dates and slots available, and notified by phone for scheduling, Dietrich said.